Pure copper is widely used in electronics, construction, decoration, and art fields due to its beautiful reddish-brown color, excellent electrical conductivity, thermal conductivity, and corrosion resistance. The purposes of its surface treatment are:
1.Prevent oxidation and discoloration.
2. obtain special colors and appearances.
3. Enhance wear resistance or other physical properties.

Below are some regular surface treatments
1.Mechanical Treatment
Mainly through physical methods, abrasives are used to change the surface texture or finish.

Mechanical Polishing
- Description: Use polishing wheels and pastes to grind and polish the copper surface to achieve a mirror or brushed finish.
- Purpose: Improve glossiness and smoothness to achieve a mirror decorative effect.

Sandblasting & Brushing
- Sandblasting: Use compressed air mixed with abrasives to impact the copper surface, forming a uniform, low-gloss matte frosted texture.
- Brushing: Use abrasive belts or nylon wheels to produce unidirectional or crosswise filamentary textures on the surface.
- Purpose: Obtain a frosted texture and linear texture appearance, hide scratches, and increase surface roughness to facilitate subsequent coating adhesion.

Barrel Polishing & Vibratory Finishing
- Description: Put copper parts and abrasives into a barrel or vibratory finishing machine, and remove burrs and oxide layers through tumbling and friction to obtain a uniform luster.
- Purpose: Remove burrs and achieve a stone-washed finish; suitable for batch processing of small-sized, high-volume parts.

2.Chemical Treatment
Change the copper surface state or form a protective film through chemical reactions in solutions.
Chemical Polishing
- Description: Immerse copper parts in a specific acid solution. The acidic solution (such as a composite system of dilute sulfuric acid, phosphoric acid, hydrogen peroxide, etc.) reacts with the copper surface to dissolve the oxide layer and form a smooth and bright metal surface.
- Purpose: Achieve a mirror effect similar to mechanical polishing, suitable for workpieces with complex shapes.
Passivation
- Description: Use benzotriazole (BTA) or other passivators to react with the copper surface to form a dense, water-insoluble protective complex film.
- Purpose: This is the most commonly used and effective anti-discoloration method. It can delay the process of copper turning black and forming patina due to the action of air and moisture.
Oxidation & Antiquing
- Description: Use chemical reagents (such as potassium sulfide, ammonia water, etc.) to cover the copper surface, accelerate the oxidation or vulcanization process, and form a film layer of black, bronze, or iridescent colors.
- Purpose: Achieve an antique and vintage artistic effect, often used in crafts and decorations.
3.Electrochemical Treatment
Electroplating
- Nickel/Chromium Plating: Electroplate a layer of nickel or chromium on the copper surface to improve surface hardness, wear resistance, and corrosion resistance, with a silvery-white appearance.
- Silver/Gold Plating: In the electronics field, silver plating can ensure electrical conductivity and prevent oxidation; gold plating is used for high-end decoration and corrosion protection.
- Purpose: Improve the new physical properties and appearance of the copper surface.
Electropolishing
Description: Similar to chemical polishing, it uses the principle of anodic dissolution to preferentially dissolve micro-high points on the surface, achieving a flat and bright effect.Purpose: Obtain a brighter and flatter surface than chemical polishing with better gloss retention.
4.Coating Treatment
Varnish & Clear Coating
- Description: Spray or dip-coat a transparent organic coating, such as acrylic paint, polyurethane paint, or epoxy resin.
- Purpose: Isolate copper from air contact to prevent discoloration and maintain the original metallic luster of copper.
Baking Paint & Powder Coating
- Description: Spray and cover the copper surface with a layer of colored paint or plastic powder.
- Purpose: Obtain various colors while providing protection and decorative functions.

5.Other Special Treatments
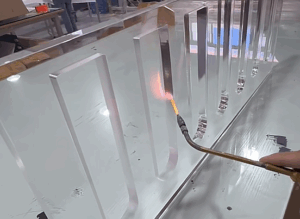
Heat Coloring
- Description: Locally or integrally heat copper with a flame or heating plate. After heating, it produces oxidation color (temper color) changes, ranging from golden yellow, purple, blue to gray.
- Purpose: Create unique artistic colors with high ornamental value.
Summary and Suggestions
| Treatment Method | Main Purpose | Appearance Effect | Advantages & Disadvantages |
| Passivation | Prevent oxidation and discoloration | Maintain original copper color, slightly darker | Most commonly used anti-corrosion method, low cost, good effect, no dimensional change |
| Chemical/Electropolishing | Improve brightness | Mirror-like brightness | Aesthetically pleasing; electropolishing offers better effect but higher cost |
| Sandblasting/Brushing | Change texture | Matte, frosted, brushed finish | Aesthetically pleasing, fingerprint-resistant, capable of hiding minor scratches |
| Clear Varnish | Prevent discoloration and retain luster | Maintain original color with film gloss | Strong protection; however, the film may age and peel off over time |
| Oxidation & Antiquing | Achieve antique artistic effect | Black, patina, iridescent, etc. | High artistic value; the surface film is relatively fragile, usually requiring clear varnish spraying for protection |
| Electroplating | Enhance wear resistance, change color, achieve high performance | Silvery white, chrome-bright, golden, etc. | Greatly improves surface performance; high cost and complex process |
How to Choose
To prevent copper discoloration: Prefer passivation or clear varnish spraying.
For high-brightness decorative effect: Choose electropolishing or mechanical polishing, followed by passivation or painting protection.
For modern industrial texture: Choose sandblasting or brushing, followed by passivation.
For antique artistic effect: Choose chemical oxidation and antiquing, followed by clear varnish spraying to fix the color.
For wear resistance or specific colors (such as silver): Choose electroplating.